Jack Crick, a 7 year old boy with a rare condition known as Severe Combined Immunodeficiency (SCID) or 'bubble boy' affecting 1 in 200,000 was treated on 25th August 2011. SCID is caused by a
mutation in a number of
genes, most commonly in the protein required for the development and differentiation of
T and B lymphocyte cells. The second most common involves a mutation that compel the body to make
abnormal enzymes called
adenosine deaminase, leading to a
reduction in immune cell production.
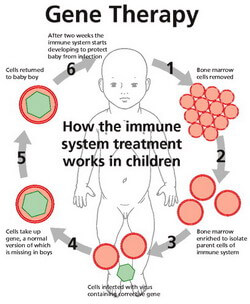
Gene therapy is used to treat SCID by removing cells from the patient's bone marrow. A viral vector is used to introduce a functioning copy of the faulty gene and the cell is re-transplanted back into the patient. This treatment is an improvement of the traditional method involving a bone marrow transplant as there is no risk of the patient's body reacting to donor material, causing a disease.
According to follow up studies published in 'Science Translational Medicine' the treatment had a success rate of 4 out of 6 for those with Adenosine Demaninase-Deficient SCID, and 10 out of 10 for those with X-linked (B and T lymphocyte) SCID.
The major drawback of the therapy is that it may activate an oncogene, which cause cancer. In the London trial for gene therapy, 1 out of the 10 patients treated for X-linked SCID developed
Leukaemia. However, it is still unclear why this occurs. Currently, new
retroviral and lentiviral vectors (tools used to deliver genetic material to cells) are being researched and developed to reduce the risk of leukaemia.
Viral Vectors
A virus is an infective agent that typically consists of a nucleic acid molecule within a protein coat. As a part of their replication cycle, all viruses attack host cells and introduce their own genetic material containing 'instructions' on how to produce more copies of the virus. Some viruses physically insert their genes into the host's genome, and these are used as viral vectors. The disease-causing gene is removed from the virus and replaced with genes encoding the desired effect depending on the treatment.
Some risks of using viral vectors are that...
- the virus may affect healthy cells as well as cancer cells
- the gene may by inserted into the wrong location of the DNA causing harmful mutations (this is what most likely occured for the X-linked SCID patients developing leukemia. The 'hematopoietic stem cells' were transduced (transferring of DNA) with a corrective transgene (exogenous gene introduced into the genome of another organism) using a retrovirus).
Ethics
As with any great medical study, there are ethical issues involved. Religious groups argue that scientists are 'playing god' by altering the way 'God' created us as gene therapy to enhance basic human traits such as height, intelligence and athletic ability is being investigated. There are also concerns that gene therapy may only be available to those who are wealthy due to it's high cost, creating a greater divide in society according to wealth.
More ethical issues concerning gene therapy can be found here. More on virus vectors can be found here.